US Slashes Testing Rules To Speedup Coronavirus Screening
Mar 17, 2020, 4:04 PM
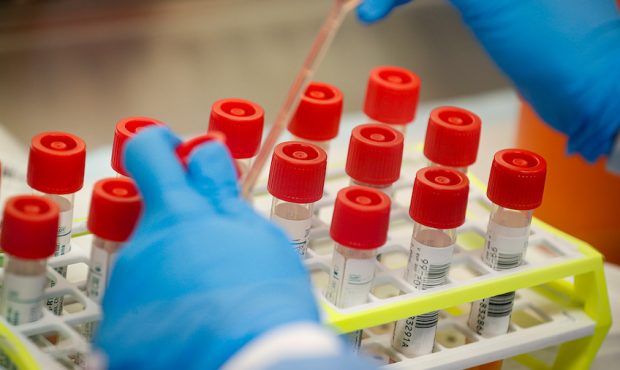
A laboratory technician prepares COVID-19 patient samples for semi-automatic testing at Northwell Health Labs, Wednesday, March 11, 2020, in Lake Success, N.Y. The US Food and Drug Administration has approved faster testing protocols as the viral outbreak continues to spread worldwide. For most people, the new coronavirus causes only mild or moderate symptoms. For some it can cause more severe illness. (AP Photo/John Minchillo)
(AP Photo/John Minchillo)
WASHINGTON (AP) — The Trump administration is slashing regulations governing test development in a bid to ramp up screening for the coronavirus amid nationwide frustration with the slow pace of the effort.
The unprecedented steps by the Food and Drug Administration could boost testing capacity at some U.S. labs, but also complicate efforts to assure the accuracy of tests and track who receives them.
More than eight weeks after the first U.S. case of COVID-19 was detected, the U.S. still struggles to conduct mass screening and provide definitive figures on the number of people tested.
Late Monday, the FDA said state health departments could essentially begin self-regulating and approving tests developed by their government-run laboratories.
“What we’re saying is that state departments of health can act as a surrogate for FDA and that the information doesn’t need to come through FDA,” FDA Commissioner Stephen Hahn told reporters on a call.
Regulators also said major test manufacturers could begin rolling out new tests without FDA pre-authorization and instead submit their applications up to 15 days after launch. Officials acknowledged the implicit trade-off of the approach, which potentially sacrifices the accuracy of unregulated tests to speed up access.
“We believe this will be a surge to meet the demand that we expect to see,” Hahn said.
For weeks, administration officials have talked about shipping millions of tests to U.S. labs. But it’s become clear those numbers have little bearing on the actual number of patients tested since most U.S. labs can process fewer than 100 samples per day. In the last week, the FDA approved the first coronavirus tests for “high-volume” laboratory systems, which are capable of processing thousands of results per day.
Efforts to tally U.S. testing figures have been stymied by the fragmented nature of the country’s health system, split between federal, state and local efforts and the private sector.
On Tuesday, members of Trump’s coronavirus task force gave the most comprehensive figures on testing yet. They estimated that 59,000 people in the U.S. have been screened for the virus since January, split roughly between government and industry labs. But they acknowledged that those figures don’t yet reflect numbers from hospitals, which should become available later this week, officials said.
Dr. Brett Giroir, the health official tasked with overseeing testing, said federal emergency personnel are working to set up nearly 50 drive-thru testing sites in 12 states this week.
“Don’t expect these to be 100% perfect the moment they come online,” Giroir told reporters.
Countries in Europe and Asia have been using drive-thru testing for weeks to screen thousands of people. South Korea, a country one-sixth the size of the U.S. in population, has reportedly tested nearly 275,000 of its citizens.
The FDA’s rush to waive testing requirements is a stark reversal from its initially cautious approach to approving tests for the virus. For almost all of February, the only FDA-authorized test was the one developed by the Centers for Disease Control and Prevention.
Even after problems with the CDC test emerged, the FDA stuck to its traditional approach of requiring labs to submit detailed testing protocols and results before winning authorization. The agency finally reversed course late last month after an outcry from state and local labs frustrated by the lack of testing options. On Feb. 29, the FDA issued new guidelines that allowed public, academic and hospital labs to develop their own in-house tests without prior government review.
In retrospect, many experts say the FDA should have taken that step earlier.
“If they fully appreciated the speed at which this would become an urgent clinical concern, they might have picked a different strategy from the beginning,” said Dr. Joshua Sharfstein, a former FDA official who is now a vice dean at Johns Hopkins University.
For most people, the new coronavirus causes only mild or moderate symptoms, such as fever and cough. For some, especially older adults and people with existing health problems, it can cause more severe illness, including pneumonia.
The vast majority of people recover. According to the World Health Organization, people with mild illness recover in about two weeks, while those with more severe illness may take three weeks to six weeks to recover.
The worldwide outbreak has sickened more than 185,000 people and killed more than 7,300.
Public health experts said Tuesday it was difficult to gauge the overall impact of the FDA’s latest regulatory changes. Outside of large states like California, New York and Washington, most states would not have the regulations or infrastructure to create and review their own tests, said a senior official for the group representing state health labs. Additionally, most state systems are already struggling to respond to the outbreak.
“All their resources right now are committed to that response,” said Eric Blank of the Association of Public Health Laboratories. “They are very lukewarm about getting involved in this altogether.”
Alta Charo, an ethicist at the University of Wisconsin at Madison who previously served as a senior FDA adviser, said the benefits of faster testing could be outweighed by inaccurate tests that “give a false sense of security” and allow an infected person to “continue mingling in the community.”
Under the latest FDA guidance, private companies would still be required to submit their testing protocols to the FDA after launch, but federal review would be waived for state labs.
Even as regulators loosened their standards for state and commercial tests, new challenges emerged.
For more than a week, lab technicians have reported shortages of chemicals and supplies needed to run the tests. Health care providers are also seeing dwindling supplies of basic equipment needed to test patients, such as swabs and protective masks.
FDA officials said they are working to find new suppliers and alternative products. Last week, the agency set up a toll-free number for lab staff seeking help with supplies.